What Happens At The Lab
We follow stringent quality control measures to ensure your baby’s stem cells are safely stored.
1. Stem Cell Processing
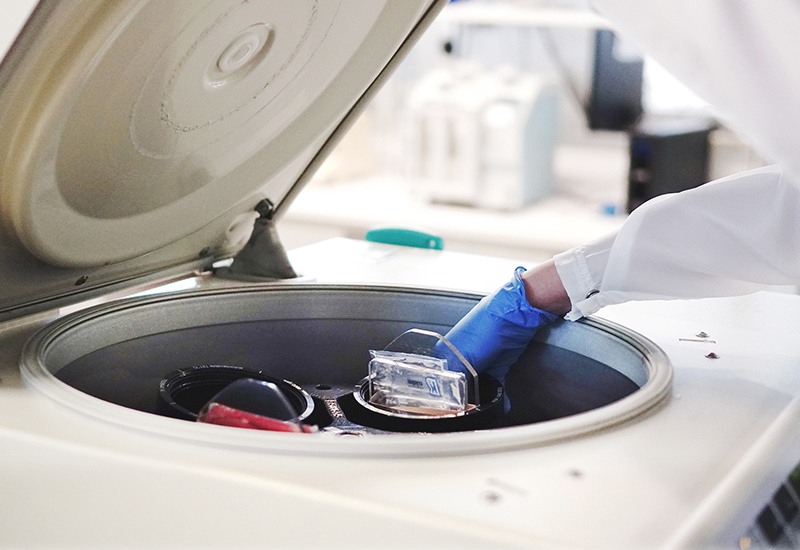
Stem cells are isolated using a special extraction procedure. This extraction process segregates red blood cells and plasma from the cord blood, leaving behind a purified and concentrated sample of stem cells for banking.
2. Sterility Check

The sterility of your child’s cord blood sample is verified to ensure that the banked specimen is sterile for future use.
3. Viability Testing

Viability assays look at the activity level of the stem cells and their ability to grow.
4. Stem Cell Processing
This test ensures that the sample, particularly cord blood, is free if infectious diseases like HIV or hepatitis.
5. Cell Count
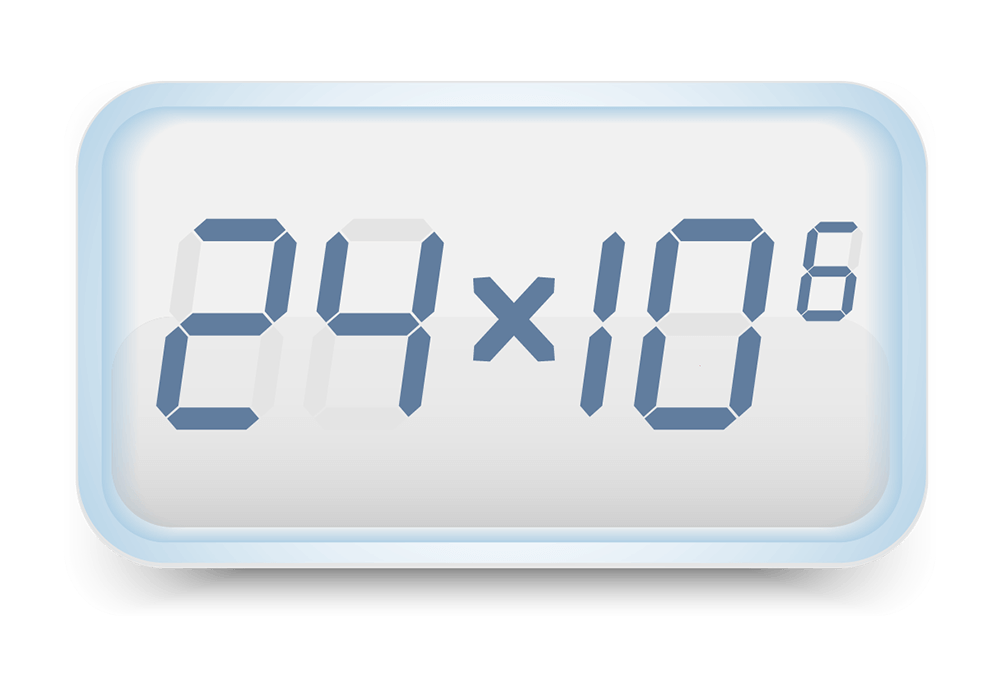
We perform cell counts on your baby’s sample before and after processing to look at cell content and components.
6. CD34+ Stem Cell Analysis

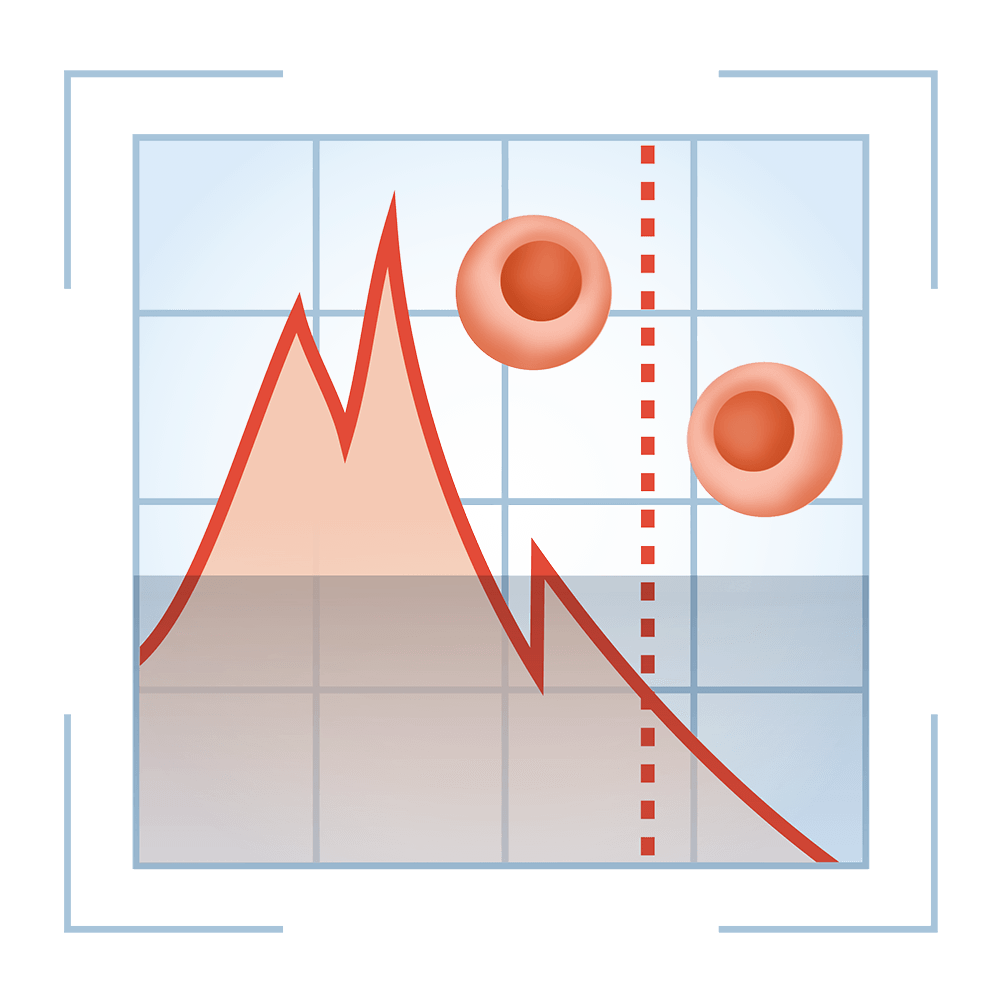
Healthcord is equipped with advanced Flow Cytometry technology to detect the number of CD34+ stem cells in your baby’s cord blood. Our single-platform enumeration system is the most accurate and advanced method available to date for quantitating CD34+ stem cells.
7. Computer Controlled-Rate Freezing

Cooling is one of the most important aspects of cryopreservation, since uncontrolled freezing can result in cell death. Our state-of-the art computer-controlled rate freezers drop the temperature in highly regulated increments, minimizing damage to the stem cells.
8. Next generation vapour phase storage

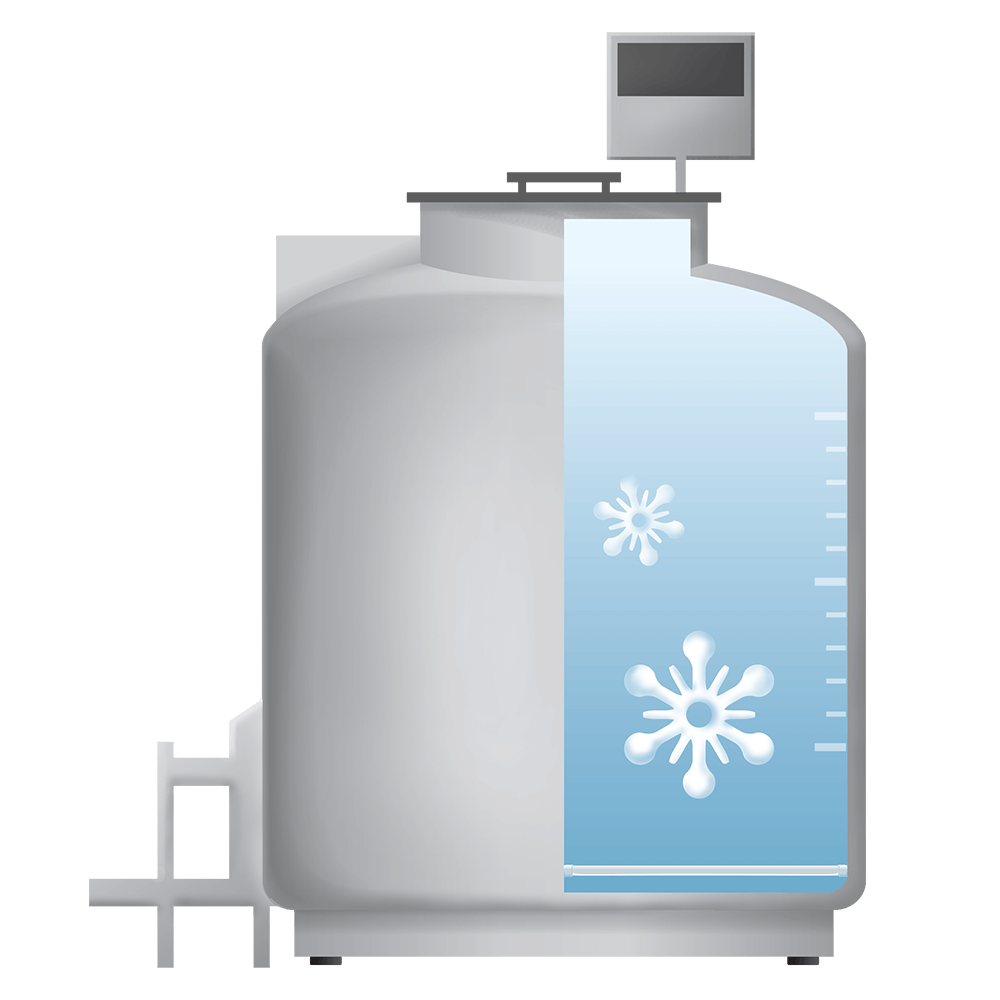
Next generation vapour phase storage is the most advanced and safest method for cryogenic storage. It eliminates the risk of contamination associated with liquid nitrogen storage tanks. We guarantee the use of vapour phase storage for all samples.
9. Certificate of banking

Once your baby’s stem cells are safely stored, you will receive a certificate of banking indicating the number of stem cells, yield, and potency along with retrieval information.
What Happens At The Lab
We follow stringent quality control measures to ensure
your baby’s stem cells are safely stored.
Stem Cell Processing


The sterility of your child’s cord blood sample is verified to ensure that the banked specimen is sterile for future use.

Stem cells are isolated using a special extraction procedure. This extraction process segregates red blood cells and plasma from the cord blood, leaving behind a purified and concentrated sample of stem cells for banking.
Sterility Check

Viability Testing
Viability assays look at the activity level of the stem cells and their ability to grow.
Cell Count
We perform cell counts on your baby’s sample before and after processing to look at cell content and components.
Stem Cell Processing
This test ensures that the sample, particularly cord blood, is free if infectious diseases like HIV or hepatitis.

Healthcord is equipped with advanced Flow Cytometry technology to detect the number of CD34+ stem cells in your baby’s cord blood. Our single-platform enumeration system is the most accurate and advanced method available to date for quantitating CD34+ stem cells.
CD34+ Stem Cell Analysis

Computer Controlled-Rate Freezing



Certificate of banking
Once your baby’s stem cells are safely stored, you will receive a certificate of banking indicating the number of stem cells, yield, and potency along with retrieval information.

Cooling is one of the most important aspects of cryopreservation, since uncontrolled freezing can result in cell death. Our state-of-the art computer-controlled rate freezers drop the temperature in highly regulated increments, minimizing damage to the stem cells.
Next generation vapour phase storage
Next generation vapour phase storage is the most advanced and safest method for cryogenic storage. It eliminates the risk of contamination associated with liquid nitrogen storage tanks. We guarantee the use of vapour phase storage for all samples.





